The world of chemistry is filled with fascinating reactions, and the interaction between silver and dilute sulfuric acid is one such captivating phenomenon. Imagine a precious metal like silver coming face-to-face with a potent acid, setting off a dance of chemistry that’s both intriguing and instructive. In this comprehensive guide, we’ll delve into the details of this reaction, exploring its history, key concepts, applications, and contemporary advancements.
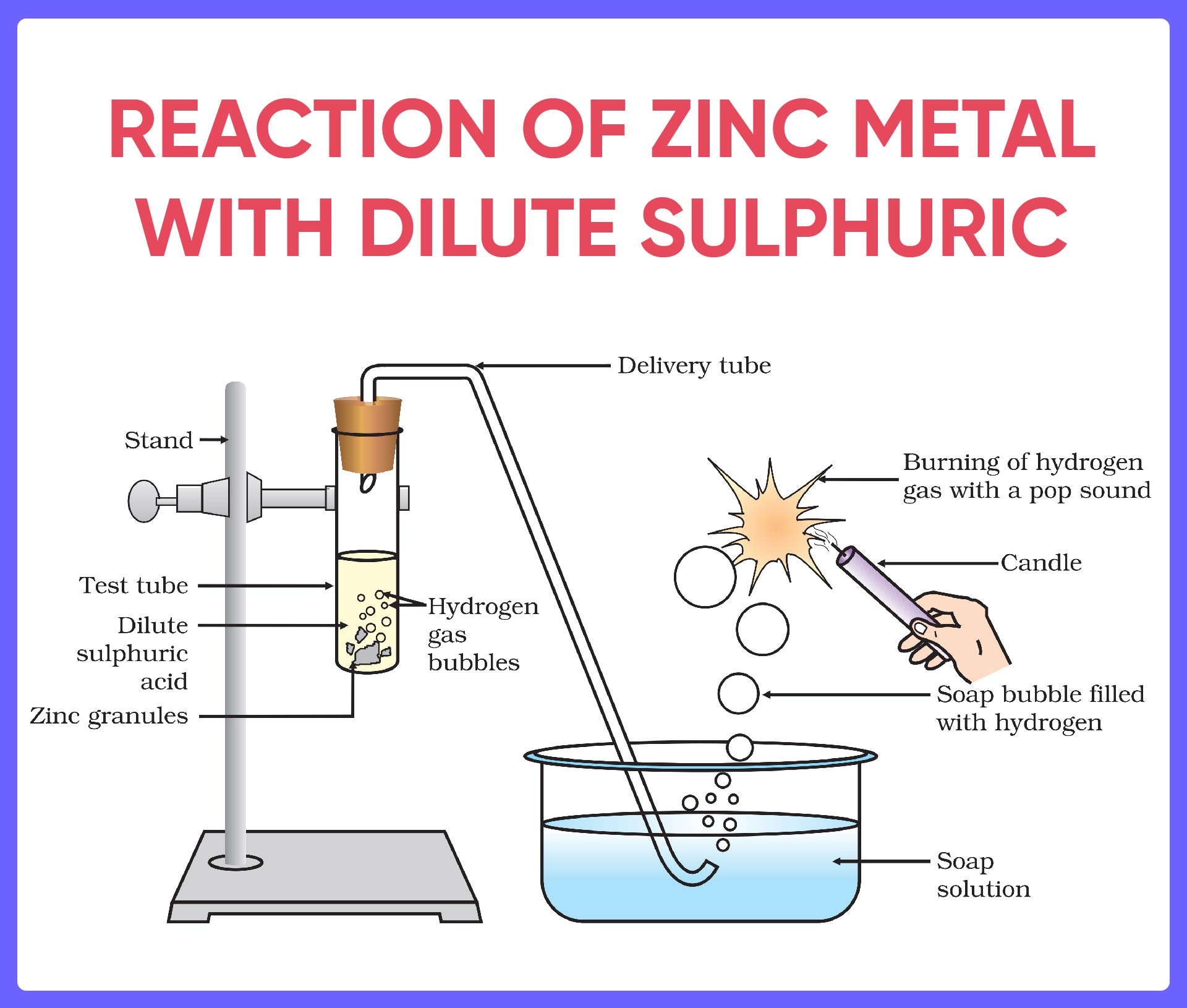
Image: www.teachoo.com
Silver and Sulfuric Acid: A Historical Retrospective
Silver, a noble metal revered for its beauty and malleability, has been a subject of scientific inquiry since antiquity. Ancient alchemists recognized silver’s exceptional properties and sought ways to manipulate them. Similarly, sulfuric acid, a strong mineral acid, has played a pivotal role in chemical processes for centuries. In the 8th century, Persian alchemist Jabir ibn Hayyan described the preparation of sulfuric acid by burning sulfur with saltpeter, marking an important milestone in acid chemistry.
The Chemical Equation: Unveiling the Reaction’s Essence
At the heart of the reaction between silver and dilute sulfuric acid lies a fundamental chemical equation:
2Ag + H2SO4 → Ag2SO4 + H2
In this equation, two atoms of silver (Ag) react with one molecule of sulfuric acid (H2SO4) to form one molecule of silver sulfate (Ag2SO4) and one molecule of hydrogen gas (H2). This balanced equation provides a quantitative understanding of the reactants and products involved in the reaction.
A Detailed Exploration of the Reaction Mechanism
To fully appreciate the reaction between silver and dilute sulfuric acid, let’s explore its mechanism step-by-step:
- Ionization: The reaction begins with the ionization of sulfuric acid in water, creating hydrogen ions (H+) and sulfate ions (SO42-):
H2SO4 + H2O → H+ + HSO4–
HSO4– + H2O → H+ + SO42-
- Reaction with Silver: The hydrogen ions produced in step 1 react with metallic silver atoms, oxidizing them to form silver ions (Ag+) and releasing hydrogen gas:
2Ag + 2H+ → 2Ag+ + H2
- Complex Formation: The silver ions produced in step 2 react with sulfate ions to form silver sulfate, a white precipitate that settles out of solution:
Ag+ + SO42- → Ag2SO4
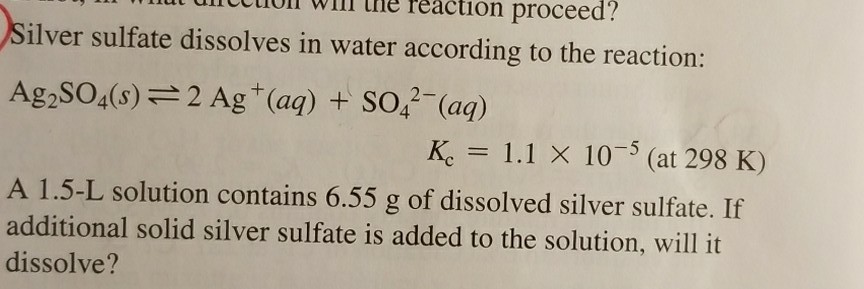
Image: www.chegg.com
Silver Sulfate: Properties, Applications, and Environmental Considerations
Silver sulfate, the primary product of the reaction between silver and dilute sulfuric acid, is a crystalline compound with distinctive properties. It is a white precipitate that is insoluble in water and only sparingly soluble in acids. Silver sulfate finds use in various industrial applications, including:
–Silver plating: Silver sulfate is used as an electrolyte in the process of electroplating, where a layer of silver is deposited on a metal surface for decorative or protective purposes.
–Photographic emulsions: Silver sulfate is a component of photographic emulsions, which are light-sensitive materials used in traditional photography.
–Antimicrobial agent: Silver sulfate possesses antimicrobial properties and thus finds use in certain medical applications, including eye drops and topical ointments.
Safety Precautions while Working with Silver and Sulfuric Acid
While the reaction between silver and dilute sulfuric acid is a fascinating scientific phenomenon, it is important to note the associated safety considerations. Sulfuric acid is a corrosive acid that can cause severe burns upon contact with skin or eyes. Proper protective equipment, including gloves, protective eyewear, and a lab coat, should always be worn when handling sulfuric acid.
Latest Developments and Future Prospects in Silver-Sulfuric Acid Chemistry
The chemistry of silver and sulfuric acid continues to be a subject of active research, with scientists investigating novel applications and innovative technologies. One such area of exploration lies in the development of silver-sulfuric acid batteries, which hold promise for storing clean and renewable energy. Researchers are also seeking to create more efficient and environmentally friendly methods for producing and utilizing silver sulfate.
Would Silver React With Dilute Sulfuric Acid
Conclusion
The reaction between silver and dilute sulfuric acid is a testament to the captivating power of chemistry, demonstrating the interactions between different elements and the dance of chemical reactions. Understanding this reaction not only provides insights into fundamental chemical principles but also yields practical applications in various industries. With ongoing research and advancements, the future holds exciting prospects for silver-sulfuric acid chemistry, with the potential to unlock novel technologies and contribute to a more sustainable world.

