As I was gazing at the vibrant hues of the sunset, a question crossed my mind: what is the secret behind the myriad of colors we perceive? Little did I know that this seemingly simple inquiry would lead me down a fascinating path, unraveling the intricate relationship between energy and wavelength.
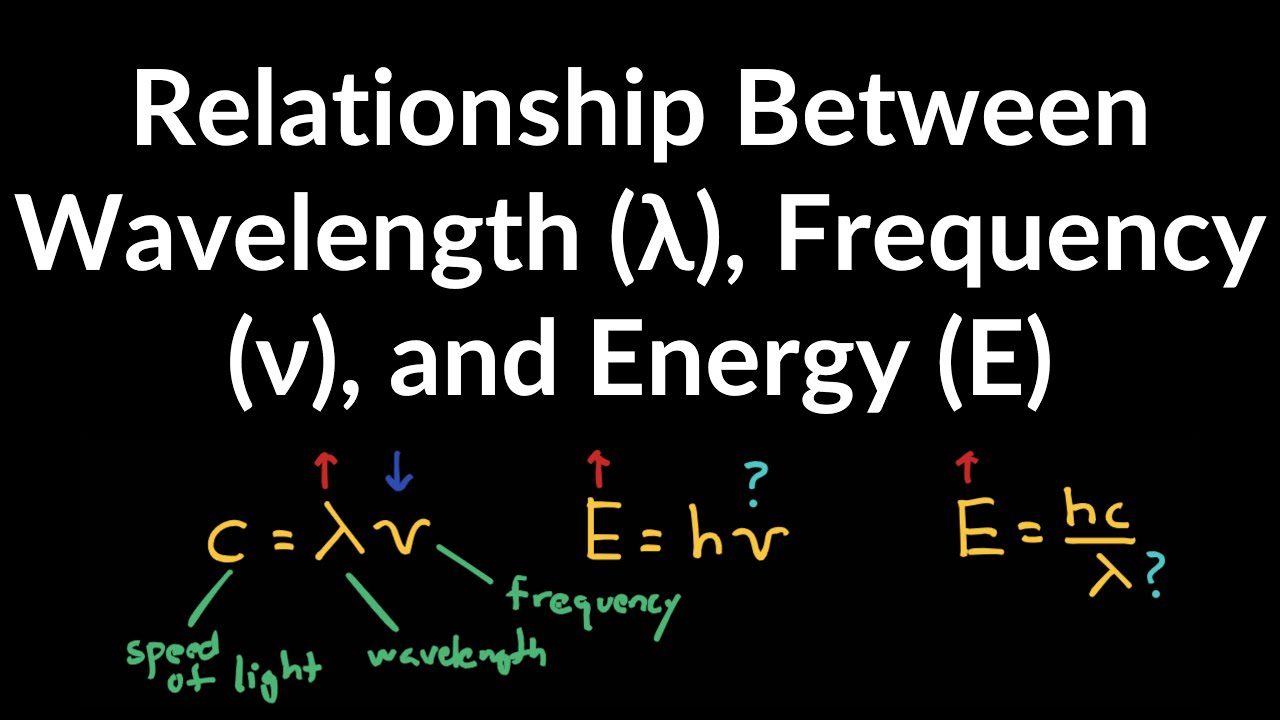
Image: ar.inspiredpencil.com
Our world is awash with an unseen force: electromagnetic radiation. This enigmatic phenomenon encompasses a spectrum of wavelengths, from the imperceptible radio waves to the formidable gamma rays, with visible light Occupying a narrow band in between. It is the wavelength that determines the form of electromagnetic radiation we experience, from the warmth of infrared rays to the penetrating power of X-rays.
Defining the Electromagnetic Spectrum
To fully grasp the relationship between energy and wavelength, let us delve into the nature of photons, the fundamental units of electromagnetic radiation. Each photon possesses a specific amount of energy, directly proportional to its frequency. Frequency, in turn, is inversely proportional to wavelength, meaning that shorter wavelengths correspond to higher energies.
Thus, the electromagnetic spectrum can be visualized as a hierarchy of energy levels. Gamma rays, with their extremely short wavelengths, wield the highest energy, followed by X-rays, ultraviolet radiation, visible light, infrared radiation, microwaves, and radio waves, in descending order of energy.
Manifestations of the Energy-Wavelength Connection
The relationship between energy and wavelength has profound implications in countless physical processes and phenomena. For instance, it governs the color of objects we see. The human eye is receptive to a limited range of wavelengths, which we perceive as different colors. Shorter wavelengths, like those of blue light, possess higher energy and stimulate the blue-sensitive cones in our retinas, while longer wavelengths, such as those of red light, have lower energy and activate the red-sensitive cones.
Another striking illustration of this connection is in the field of particle physics. The energy of photons bears direct consequences for their ability to interact with matter. Higher-energy photons, like X-rays, can penetrate deep into materials, enabling them to generate detailed medical images. In contrast, lower-energy photons, like radio waves, have difficulty penetrating dense substances, but excel in applications like wireless communication.
Understanding the Latest Trends and Developments
Recent advancements in photonics have pushed the boundaries of our knowledge and technological capabilities. Researchers are exploring novel ways to harness and manipulate photons for a wide array of applications. One such area of promise is the development of ultra high-energy photons, which could revolutionize cancer treatment by precisely targeting and destroying tumor cells.
In the realm of telecommunication, scientists are exploiting the wavelength diversity within the electromagnetic spectrum to enhance data transmission capacities. By employing multiple wavelengths simultaneously, they can achieve significantly higher bandwidths and enable faster internet speeds.

Image: energywavetheory.com
Expert Advice for Maximizing Understanding
For those aspiring to delve deeper into the fascinating world of energy and wavelength, here are some expert insights to facilitate your journey:
- Start with a strong foundation in the basic principles of physics, including notions like energy, frequency, and wavelength.
- Explore online resources and textbooks that provide comprehensive overviews of the electromagnetic spectrum and its applications.
Regularly engage with forums and online communities where discussions on these topics thrive. This exposure to diverse perspectives and expert knowledge will greatly enrich your understanding.
Frequently Asked Questions
To further enhance your comprehension, let’s tackle some commonly asked questions:
- How does wavelength affect the behavior of light?
Wavelength determines whether light bends when passing through different materials (refraction), reflects off surfaces, or undergoes interference
- What are the practical applications of different wavelengths?
Visible light is crucial for vision, X-rays are used in medical imaging, radio waves enable wireless communication, and microwaves facilitate cooking
What Is The Relationship Between Energy And Wavelength
https://youtube.com/watch?v=XH_XWHHJwUc
Conclusion
The relationship between energy and wavelength is a fundamental aspect of the electromagnetic spectrum, profoundly influencing the very fabric of our universe. From the vibrant colors we behold to the advanced technological advancements we harness, this connection plays a pivotal role in our perception and interaction with the world around us.
Now the question, are you ready to delve further into the captivating domains of energy, wavelength, and photons?

