An Excitation to Discover the Microscopic Realm of Chemistry

Image: www.slideserve.com
A chemical equation captures the intricate dance of elements and molecules undergoing transformation. While the reactants and products hold the spotlight in this play, there exist smaller players with a significant role to play: subscripts. These seemingly tiny numbers tucked beneath element symbols carry immense power, influencing the balancing of the equation and unveiling the underlying stoichiometry.
Unveiling the Secrets of Subscripts: An Essential Guide
In the realm of chemistry, balance is paramount, like a carefully choreographed symphony. Subscripts ensure this harmony by specifying the exact number of atoms involved in each molecule. Delve into the enthralling world of subscripts and uncover the pivotal part they play in unveiling the hidden order within chemical reactions.
The Significance of Subscripts in Stoichiometry
Stoichiometry delves into the intricate relationships between reactants and products, exploring their relative proportions. Subscripts serve as the guiding light in this endeavor. By precisely defining the number of atoms in each species, they empower chemists to calculate the exact amounts of reactants and products required for balanced equations.
Historical Perspectives on Subscripts
The concept of subscripts has its roots in John Dalton’s pioneering atomic theory. Dalton proposed that elements exist as individual particles, and that these particles combine in specific ratios to form compounds. Subscripts emerged as a tool to represent these ratios, laying the foundation for the precise understanding of chemical reactions.
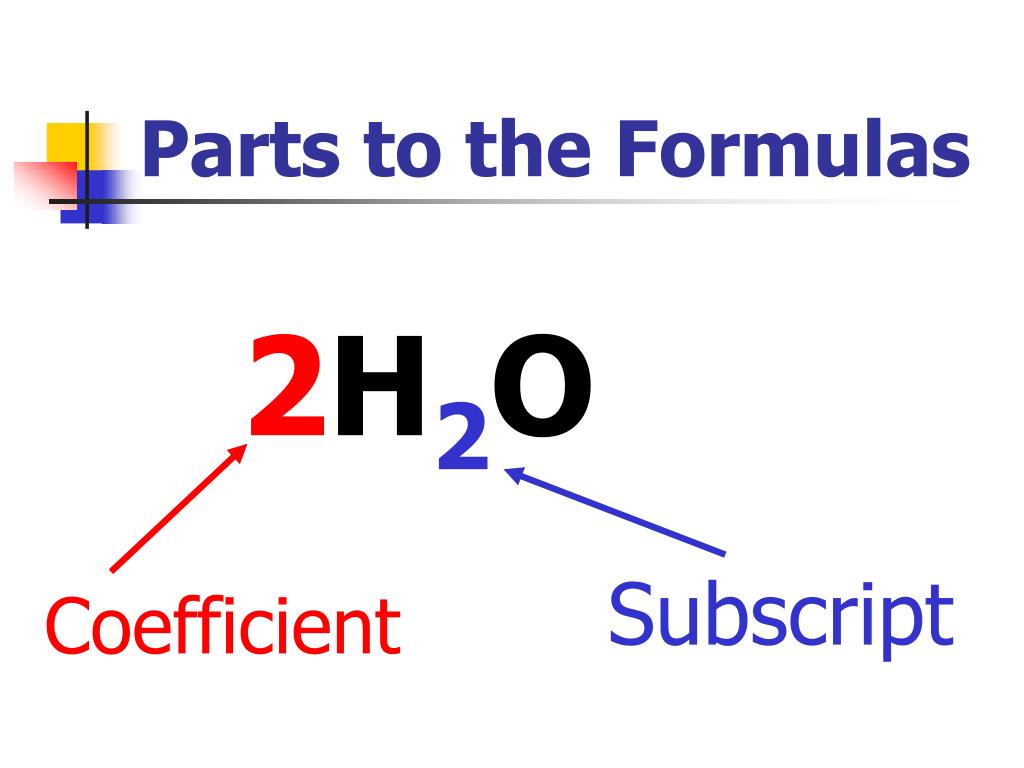
Image: www.slideserve.com
Unraveling Chemical Equations
Consider the reaction between hydrogen and oxygen to form water:
2H2 + O2 → 2H2O
The subscript “2” attached to H2 indicates two molecules of hydrogen gas (containing two hydrogen atoms each), while the subscript “1” beneath O2 signifies one molecule of oxygen gas (containing two oxygen atoms). These numbers ensure that atoms are neither created nor destroyed during the reaction.
Beyond Elements: The Versatility of Subscripts
Subscripts extend their influence beyond elements to encompass charged species, known as ions. A subscript placed after the charge symbol of an ion indicates the magnitude and type of charge associated with it. For instance, Na+ represents a sodium ion with a single positive charge.
Practical Implications: The Role of Subscripts in Chemical Analysis
In chemical analysis, determining the composition of unknown substances becomes crucial. Subscripts provide invaluable information by indicating the atomic ratios in molecules, allowing chemists to decipher the structure and properties of a given compound.
Exploring the Applications of Subscripts
Subscripts find diverse applications in chemistry, including:
- Balancing chemical equations
- Calculating the mass ratios of reactants and products
- Determining the molar ratios between substances in a reaction
- Analyzing the structure of molecules
- Predicting the properties of chemical compounds
What Is A Subscript In A Chemical Equation
Embracing the Microscopic Realm: A Final Glimpse
Subscripts hold tremendous power in revealing the intricate world of chemical equations. Their role in stoichiometry and the unraveling of chemical reactions highlights the importance of capturing every detail in the grand symphony of chemical processes. As explorers in this microscopic realm, we cherish the ability to decipher the hidden order and beauty enshrined within these equations.

