Proteins, the building blocks of life, are dynamic molecules that dance a complex tango of shapes. At the heart of this intricate choreography lies the secondary structure, a pivotal determinant of their function. Proteins undergo a series of precise folds and coils, shaping themselves into intricate structures that enable them to perform specific biological tasks.
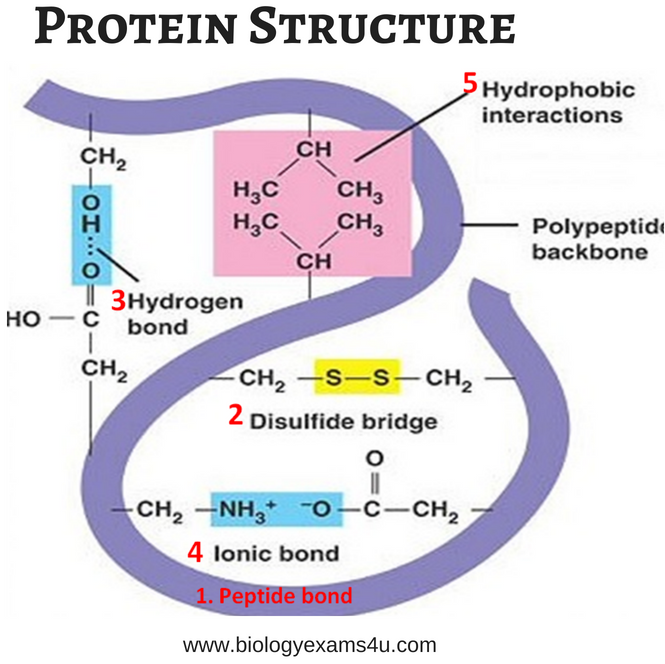
Image: ar.inspiredpencil.com
To uncover the secrets of these ethereal conformations, we embark on a journey into the secondary structure of proteins, examining the forces that sculpt their forms and the profound implications for their function.
The Alpha Helix: A Right-Twirling Tapestry
In the realm of proteins, the alpha helix emerges as a spiraling ribbon, its backbone forming a right-handed twist. The peptide bonds, like tiny hinges, orchestrate a smooth, helical dance, stabilized by hydrogen bonds between adjacent turns. This elegant coil is commonly found in transmembrane domains, playing a crucial role in protein-protein recognition and membrane dynamics.
The Beta Sheet: A Parallel or Antiparallel Rendezvous
Unlike the helix’s continuous spiral, the beta sheet unfolds as a series of adjacent strands, arranged either parallel or antiparallel to one another. These strands interact intimately through hydrogen bonds, forming pleated sheets that contribute to the structural integrity of proteins. Beta sheets are prevalent in enzymes, endowing them with the rigidity and stability necessary for catalytic prowess.
The Power of Interactions: Hydrogen Bonds Stabilize the Show
Hydrogen bonds, the invisible architects of secondary structure, orchestrate the precise placement of amino acid residues, influencing the protein’s overall shape and stability. These weak but essential interactions determine the orientation of backbone atoms, ensuring the proper formation and maintenance of helices and sheets.
Image: slidetodoc.com
Unveiling the Mechanics of Protein Folding
The formation of secondary structures is a highly orchestrated affair, governed by a multitude of forces. Van der Waals interactions, hydrophobic interactions, disulfide bonds, and electrostatic interactions collaborate to create an intricate energy landscape, guiding proteins towards their specific folded states. These forces, acting in concert, fine-tune the molecular dance, ensuring the protein’s functional integrity.
The journey from a linear chain of amino acids to a precisely folded protein molecule is a breathtaking testament to the marvels of nature’s biochemistry. As we deepen our understanding of these intricate mechanisms, we not only unlock insights into the enigmatic world of proteins but also pave the way for breakthroughs in biotechnology, disease diagnosis, and therapeutics.
Latest Trends and Developments
The constant pursuit of knowledge about protein secondary structures has led to significant advancements. Recent research has shed light on the dynamics of these conformational transitions, revealing the fluidity of these structures under physiological conditions. Furthermore, novel techniques, such as Förster resonance energy transfer (FRET) microscopy and time-resolved fluorescence anisotropy, empower researchers to probe the structural changes in real time.
Empowering Health and Innovation: Practical Tips for Engaging with Proteomics
With the surge in proteomic research, understanding protein secondary structures has become imperative for uncovering their biological significance. To delve deeper into this fascinating realm, consider these insightful tips:
- Immerse Yourself in Bioinformatics: Bioinformatics tools, such as online databases and software, offer invaluable aid in studying protein sequences, structures, and interactions.
- Embrace Structural Biology Techniques: Techniques like X-ray crystallography, NMR spectroscopy, and cryo-electron microscopy provide detailed insights into protein structures, enabling researchers to visualize the intricate spatial arrangements of atoms.
- Consult with Experts: Seek guidance from experts in the field of protein biochemistry and structural biology. Their insights and experience can significantly enhance your understanding and research endeavors.
By adopting these tips, you empower yourself with the tools and knowledge to navigate the complex world of proteomics, paving the way for groundbreaking discoveries and transformative applications in healthcare and biotechnology.
FAQs: Unveiling the Mysteries of Protein Secondary Structures
- Q: What is the primary determinant of alpha helix and beta sheet formation?
A: The sequence of amino acids, particularly the polarity of their side chains, primarily drives the formation of helical and beta sheet structures. - Q: Can secondary structures spontaneously convert between alpha helix and beta sheet?
A: While spontaneous conversion is possible, it is relatively rare. Typically, a significant conformational change, facilitated by external factors or chaperone proteins, is required for such transitions. - Q: How do secondary structures contribute to protein function?
A: Secondary structures provide stability, flexibility, and specific binding interfaces, enabling proteins to interact with other molecules, catalyze biochemical reactions, and perform diverse cellular tasks.
The Secondary Structure Of A Protein Results From _____.
Conclusion: Unveiling the Symphony of Life
Protein secondary structures, the intricate building blocks of life’s machinery, stand as a testament to the exceptional sophistication of nature’s design. Through their molecular ballet, proteins enact a symphony of biological functions, underpinning cellular processes, orchestrating biochemical reactions, and shaping the delicate balance of life. As we delve deeper into the enigmatic world of proteomics, we unlock not only a profound appreciation for the intricate harmony of molecular biology but also the potential to unravel new frontiers of scientific inquiry and harness the power of proteins to address unmet medical challenges.
Are you passionate about the captivating world of proteins and their secondary structures? Share your thoughts below and join the ongoing conversation!

