What is Specific Latent Heat of Fusion: Delving into the Essence of Phase Transitions
The world around us is constantly transforming, with matter transitioning between different states—from solids to liquids to gases and back again. At the heart of these transformations lies a fundamental concept known as specific latent heat of fusion. It represents the precise amount of energy required to convert a solid substance into a liquid, at a constant temperature. Understanding this concept is crucial for comprehending numerous physical phenomena and technological applications.
Defining Specific Latent Heat of Fusion
In essence, the specific latent heat of fusion is the quantity of heat energy that must be absorbed by one kilogram of a solid substance to undergo a complete phase transition from a solid to a liquid state, without any change in temperature. This energy is utilized to overcome the intermolecular forces that hold the solid particles fixed in a rigid structure, allowing them to become mobile and flow freely as a liquid. Each substance possesses a unique specific latent heat of fusion, reflecting the strength of its intermolecular interactions.
Phase Transitions and Energy Transfer
Phase transitions, such as melting and freezing, involve energy transfer without a change in temperature. During melting, heat energy is absorbed, breaking the intermolecular bonds and transforming the solid into a liquid. Conversely, when a liquid freezes, it releases the same amount of heat energy, solidifying back into a rigid structure. This reversible process highlights the dynamic nature of matter and the role of energy in shaping its physical properties.
Applications of Specific Latent Heat of Fusion
The concept of specific latent heat of fusion finds myriad applications in various scientific and industrial domains. One notable example is in the design of thermal energy storage systems. Materials with high specific latent heat of fusion can be used as thermal storage media, absorbing large amounts of heat energy during phase transitions. This stored energy can then be released gradually, providing a cost-effective and environmentally sustainable way to regulate temperature in buildings and industrial processes.
Another important application lies in cryogenics, the study of extremely low temperatures. By understanding the specific latent heat of fusion of different substances, scientists can design advanced cooling systems and optimize cryogenic freezing techniques, enabling breakthroughs in fields such as medicine and space exploration.
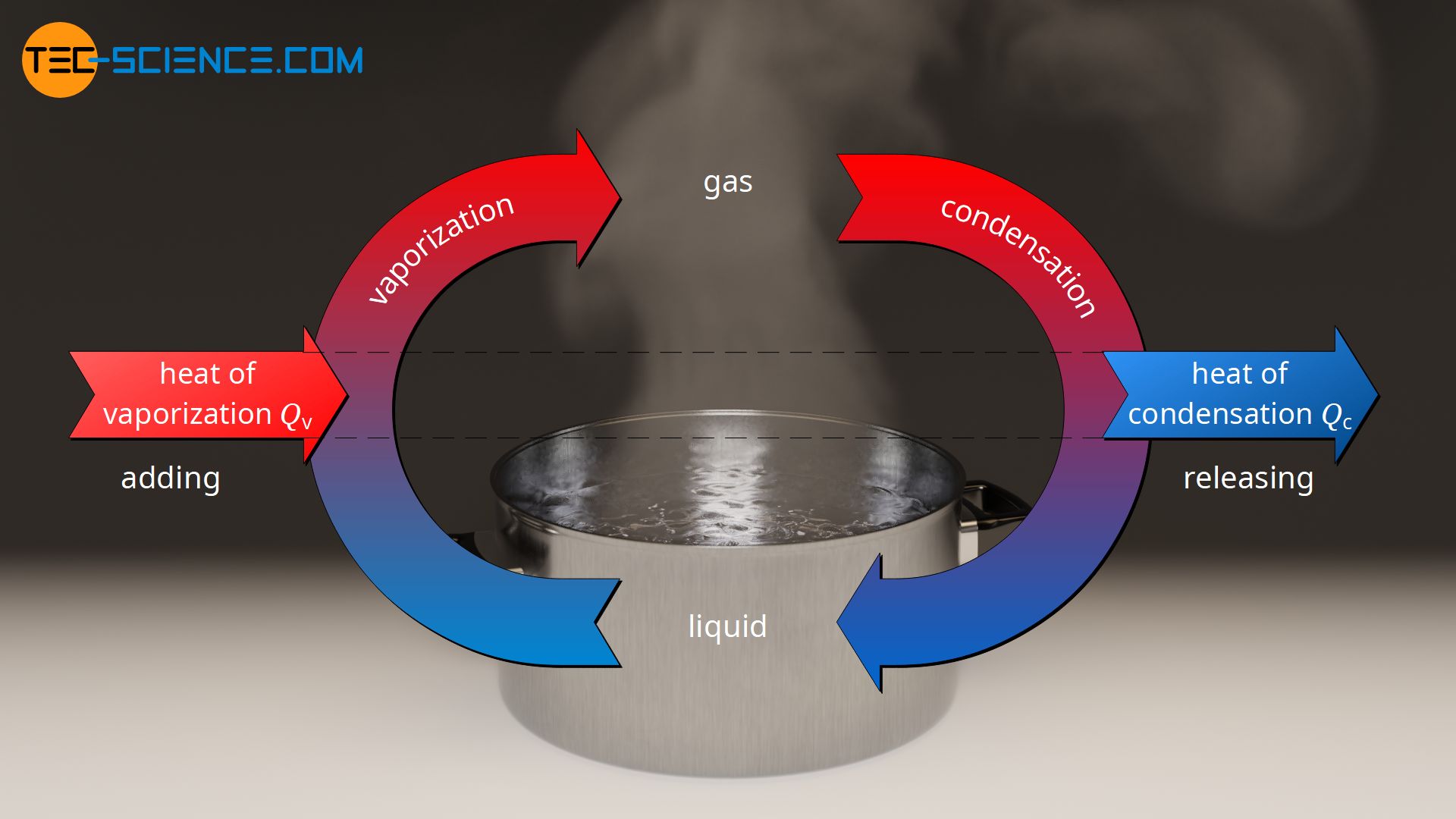
Image: www.tec-science.com
Exploring Real-World Examples
To illustrate the significance of specific latent heat of fusion, let’s delve into some practical examples. The melting of ice at 0°C is a familiar phenomenon. To melt one kilogram of ice fully, 334 kilojoules of heat energy must be absorbed. This energy breaks the hydrogen bonds between water molecules, transforming them from a rigid lattice structure to a liquid state.
Another instance is the solidification of molten metals in casting and welding processes. By controlling the specific latent heat of fusion of the metal, engineers can optimize the cooling rate and obtain desired material properties for specific applications. Understanding this concept empowers them to design stronger and more efficient structures.
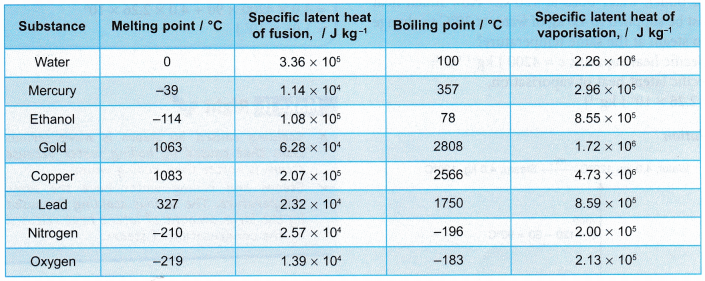
Image: cbselibrary.com
What Is Specific Latent Heat Of Fusion
The Significance of Understanding Specific Latent Heat of Fusion
Grasping the concept of specific latent heat of fusion is fundamental for comprehending diverse physical phenomena and technological advancements. It unveils the energetic foundations of phase transitions, energy storage mechanisms, and numerous applications in various fields. By delving into this concept, we gain a deeper appreciation for the intricate workings of matter and the role of energy in shaping our world.

